
Scientists report world's first X-ray of a single atom։ What does it look like?
A team of scientists from Ohio University, Argonne National Laboratory, the University of Illinois-Chicago, and other prestigious institutions has achieved a groundbreaking milestone by successfully capturing the X-ray signature of a single atom. Led by Ohio University Professor of Physics and Argonne National Laboratory scientist, Saw Wai Hla, this achievement has the potential to revolutionize the field of material detection.
X-rays, discovered by Roentgen in 1895, have found widespread applications in various areas, including medical examinations and airport security screenings. Even NASA's Mars rover, Curiosity, utilizes X-ray devices to analyze the composition of rocks on Mars. In scientific research, X-rays are crucial for identifying the types of materials present in a sample.
With advancements in synchrotron X-ray sources and new instruments, the quantity of material required for X-ray detection has significantly decreased over the years. Currently, the smallest sample size that can be X-rayed is on the order of attograms, equivalent to around 10,000 atoms or more. However, the weak X-ray signal produced by a single atom poses a challenge for conventional detectors, rendering it undetectable. Hla and his research team have now accomplished the long-standing goal of capturing the X-ray signature of a single atom.
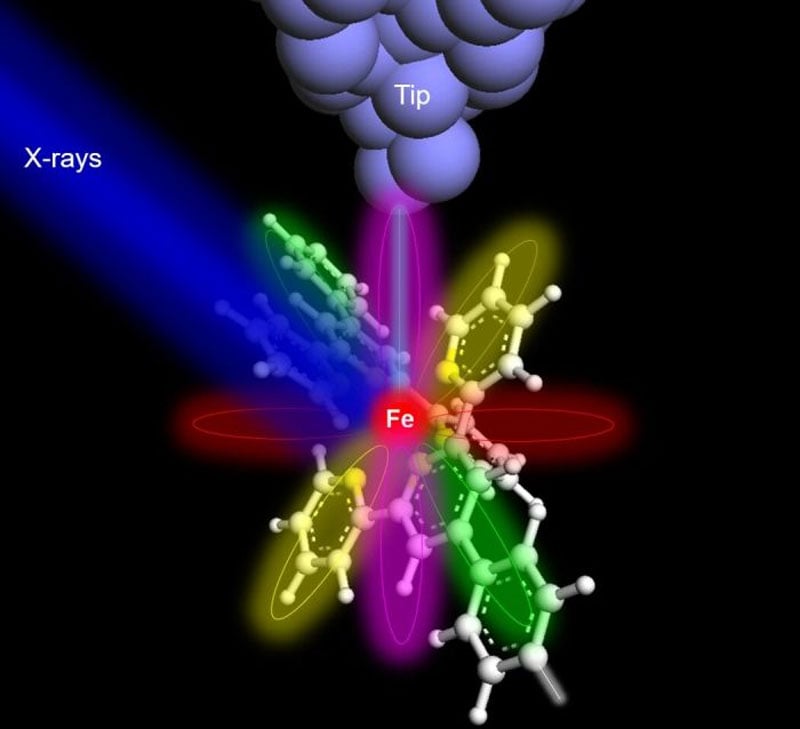
Hla, who also serves as the director of the Nanoscale and Quantum Phenomena Institute at Ohio University, explained the significance of their breakthrough: "Atoms can be routinely imaged with scanning probe microscopes, but without X-rays, one cannot determine their composition. We can now precisely identify the type and chemical state of a particular atom, one atom at a time. This achievement will have a profound impact on environmental and medical sciences, and it may even lead to groundbreaking cures with far-reaching implications for humankind. This discovery is poised to transform the world."
The team's research, detailed in a paper published in the renowned scientific journal Nature on May 31, 2023, and featured on the cover of the print version on June 1, 2023, outlines their methodology. Hla and his team of physicists and chemists, including Ph.D. students from Ohio University, utilized a purpose-built synchrotron X-ray instrument at the XTIP beamline of the Advanced Photon Source and the Center for Nanoscale Materials at Argonne National Laboratory.
To demonstrate the efficacy of their technique, the researchers selected an iron atom and a terbium atom, each embedded within molecular hosts. To detect the X-ray signal from a single atom, the team augmented conventional X-ray detectors with a specialized detector consisting of a sharp metal tip positioned in close proximity to the sample. This setup allowed for the collection of X-ray excited electrons, employing a technique known as synchrotron X-ray scanning tunneling microscopy or SX-STM. X-ray spectroscopy in SX-STM is triggered by the photoabsorption of core-level electrons, which provides elemental fingerprints, enabling direct identification of the elemental composition of materials.
Hla described the obtained X-ray spectra as unique fingerprints, each capable of precisely determining the identity of a specific atom. This breakthrough technique opens up new possibilities for research and the development of technologies in fields such as quantum information, the detection of trace elements in environmental and medical research, and advanced materials science instrumentation.
Tolulope Michael Ajayi, the first author of the paper and a Ph.D. student, commented on the significance of the research: "The technique and concept proven in this study break new ground in X-ray science and nanoscale studies. Using X-rays to detect and characterize individual atoms could revolutionize research and spawn new technologies in various fields, including quantum information and environmental and medical research involving trace elements
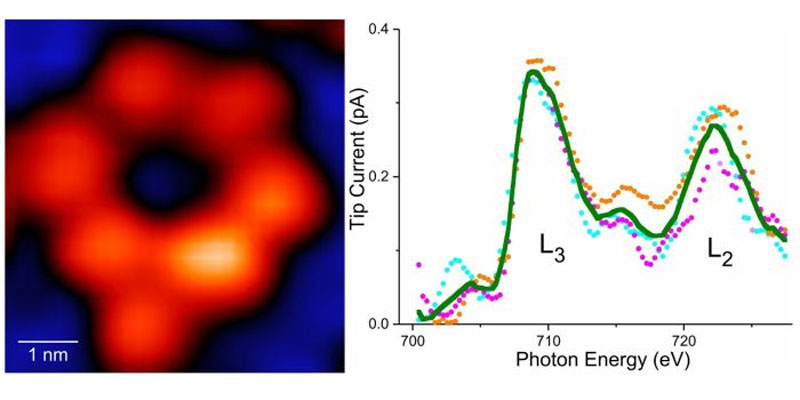
On the left is a molecule with a single atom of iron, and on the right is a curve passing through a detector that detects iron and its chemical activity.
- Related News
- Scientists create optical-mechanical quantum memory that can become basis of quantum Internet
- The water found on Moon is enough for about 1 million people - Vladimir Surdin
- There may be life on Venus, claims new research
- Powerful sun flare triggers rare phenomenon that was last seen in 1942
- Planet not far from Earth could potentially be habitable ocean-planet
- China launches satellite into lunar orbit to help deliver soil sampled from far side of Moon
- Most read
month
week
day
- iPhone users are advised to disable iMessage: What risks are hidden in it? 1476
- Problems with Android 15: NFC contactless payments no longer work on smartphones with updated operating systems 1177
- Pavel Durov gives interview to Tucker Carlson: From 3-hour interview, less than hour appears in final version 1086
- AMD Ryzen 7 processor, 24 GB of RAM and only $550: Mechrevo presents inexpensive and powerful laptop (photo) 824
- Black Shark smart ring from Xiaomi to have interesting characteristics and phenomenal autonomy: 180 days of operation without recharging (photo) 786
- The 5 most controversial buildings ever built: Bold design or complete failure? (photo) 785
- Armenia takes 89th place in terms of mobile Internet speed, but leads region in terms of fixed line speed 693
- 3 best smartphones under $160 665
- Telegram receives major update and sticker editor 649
- No internet access required? How AI will work in iPhone 570
- Archive
